HIV (Human Immunodeficiency Virus) directly damages the immune system when it enters the human body. The immune system tries to fight the infection but the body does not show any symptoms in the initial stage, thereby silently killing most of the cells associated with the immune system i.e. CD4 cells. This causes various other infections in the body and may also lead to cancer. The HIV is transmitted mainly through bodily fluids that include blood, semen, and breast milk, vaginal and rectal fluids.
HIV is a lifelong condition and currently, there is no permanent treatment for it. However, HIV infection can be treated in the initial stages. An HIV positive person if not treated can develop a serious condition called AIDS (acquired immune deficiency syndrome). In this stage, the body’s immune system becomes too weak and is unable to fight off other diseases or infections and drastically reduces the life expectancy of a person to 3 years. Whereas, an HIV infected person with proper treatment can have a life expectancy same as a person with no HIV infection. Many therapies and drugs have been developed for fighting HIV/AIDS, but there is no permanent cure as of now.
Recently, the U.S. Food and Drug Administration has given approval to a new drug called Bictegravir for treating HIV infections. Bictegravir belongs to a group of HIV drugs called integrase inhibitors. It blocks an HIV enzyme known as integrase and prevents the infection from multiplying.
Concert Pharmaceuticals, a clinical stage biopharmaceutical company, recently got published a new patent application no WO2018005328A1titled as “Deuterated Bictegravir”. This patent application discloses the deuterated forms of Bictegravir and its pharmaceutically acceptable salts thereof with below mentioned formula and its method of administration.
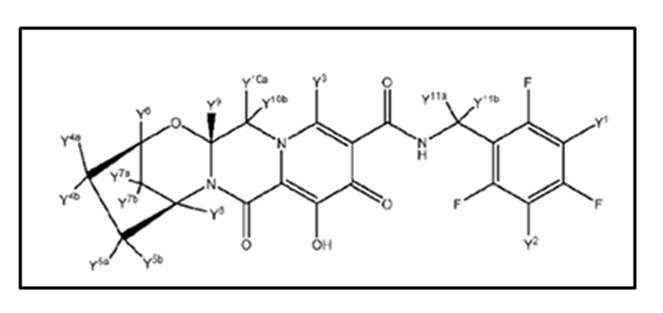
The patent application in one embodiment discloses the use of Bictegravir in methods of treating diseases and conditions related to HIV infection. It also provides various methods to inhibit the activity of HIV infection enzyme called integrase. The chemical name of Bictegravir is (2R,5S,13aR)-8-hydroxy-7,9-dioxo-N-(2,4,6-trifluorobenzyl)-2,3,4,5,7,9,13-,13a-octahydro-2,5-methanopyrido[1′,2′:4,5] pyrazino[2,1-b][1,3]oxazepine-1-0-carboxamide. Code names of Bictegravir are S-9883 and GS-9883-001, they are potent inhibitors of integrase that rapidly reduce HIV infection in humans.
According to the patent application, Bictegravir is currently under Phase III clinical trials for the treatment of HIV-1 infection and it has been tested for safety efficacy in the antiretroviral treatment of HIV infected adults as a fixed combination with emtricitabine/tenofovir alafenamide.
In another embodiment, the patent application also discloses pharmaceutical compositions that comprise an effective amount of the disclosed compound and a pharmaceutically acceptable carrier like ion exchangers, alumina, aluminum stearate, lecithin, serum proteins, such as human serum albumin, buffer substances such as phosphates (e.g., phosphate-buffered saline, etc.). It can be administered orally and available as discrete units like capsules, sachets,tablets (which contains a predetermined amount of compound), powder or granules, solution or suspension in a liquid. The compound can also be administered in the form of suppositories for rectal administration or nasal aerosol or inhalation.
Although the HIV infection or AIDS cannot be fully cured but the compositions of Bictegravir explained in this patent are highly effective in reducing the HIV infection quickly therefore preventing the HIV infection from spreading into the whole body thereby increasing the life expectancy of patients.

Appreciating the dedication you put into your site and in-depth information you provide. It’s nice to come across a blog every once in a while that isn’t the same out of date rehashed information. Wonderful read! I’ve bookmarked your site and I’m including your RSS feeds to my Google account.