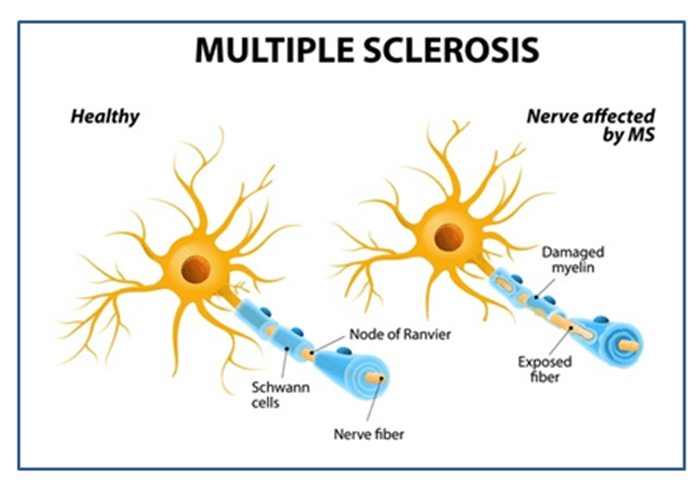
Autoimmune diseases are immune diseases that result from an error made by the immune system of the body. They have a very complex and difficult treatment methodology. In this condition, body’s immune system instead of defending against diseases, attacks the healthy cells in the body as it recognizes them as foreign invaders. Autoimmune diseases are often difficult to diagnose. One of the most common autoimmune diseases is Multiple sclerosis (MS). Multiple sclerosis is a chronic autoimmune and inflammatory neurological disease of the central nervous system. It can affect patient’s brain, spinal cord and optic nerves in the eyes causing problem in the balance, muscle control and the other body functions.
For the treatment of MS, early diagnosis is important because its treatment is slow. For diagnosis, MRI, spinal taps or blood tests are conducted. Most common symptoms of Multiple sclerosis MS are;
- Muscle weakness
- Numbness and tingling in the body
- Lhermitte’s sign (sensation like electric shock during movement of the body)
- Bladder problems
- Bowel problems
- Fatigue
- Dizziness and vertigo
- Sexual dysfunction
- Spasticity and muscle spasms
- Vision problem
- Emotional changes and depression
- Learning and memory problem
Treatment – There is no cure for MS but to slow down the progression of MS various medications and vaccines have been developed. Recently in 2019, a new drug has been approved that is Mayzent (with the active ingredient being Siponimod) for the treatment of autoimmune disease like Multiple sclerosis.
The ‘Novartis AG’ assigned new patent application no. US20170027907A1titled “Sip modulator immediate release dosage regimen” discloses the use of a novel drug “Siponimod” (BAF312) for use in the treatment of an autoimmune disease. According to the patented invention, siponimod is used in the dosage form which comprises 2 mg siponimod which is administered once daily to the patient so as to maintain the regimen. Before that the patient has to experience a titration regimen of 0.25 mg of siponimod at day 1, 0.25 mg at day 2, 0.5 mg at day 3, 0.75 mg at day 4 and 1.25 mg at day 5.
In one of the embodiments, the dosage form of siponimod is an oral solid dosage form. In another embodiment, the dosage of siponimod is preferably a tablet and alternatively the dosage can be a capsule. According to the patent, the immediate release dosage form can be made by mixing siponimod with at least pharmaceutical excipient like lubricants (stearic acid, adipic acid, sodium stearyl fumarate and/or glyceryl behenate), fillers (plant cellulose), disintegrants, glidants and binders (saccharose, gelatine, polyvinylpyrrolidone).
Although there is no permanent cure for autoimmune diseases like Multiple sclerosis, but after Siponimod (BAF312) drug approval the progression of the disease can be slowed down to give the patient some more time. It has reduced the risk of disability progression in twenty six percent of the patients who received the drug. Another important point is that the drug works in a similar manner for both men and women.